Genetically-encoded reagents for high throughput connectomics
Type: Optics / Microscopy,
Keywords: Genetically-encoded reagent, Fluorescence-based reagent, Fluorogen-activating protein, Fluorescence-synapse labeling, Connectivity analysis
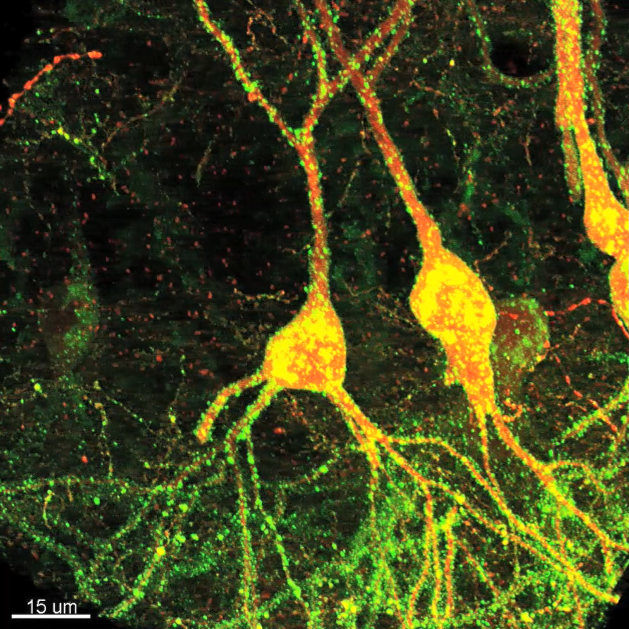
High-throughput, quantitative connectomics using fluorescence microscopy
We have developed new genetically-encoded reagents for fluorescence-synapse labeling and connectivity analysis in brain tissue designed for high-throughput, compartment-specific localization of synapses across diverse neuron types in the mammalian brain. High-resolution confocal image stacks of sparsely-labeled, virally-transduced neurons can be used for 3D reconstructions of postsynaptic cells, automated detection of synaptic puncta, and multichannel fluorescence alignment of dendrites, synapses, and presynaptic neurites to assess cell-type specific connectivity. We are using these fluorescence-based reagents to quantitatively evaluate changes in synaptic connectivity during learning and in mouse models of neurological disorders. The vast number of fluorescently-labeled, input- and target-specified synapses we are collecting offers new and exciting opportunities for data analysis and machine learning.
* Employs fluorogen-activating protein (FAP), a modified antibody fragment that emits in the far red on binding of a small molecule ligand, a derivative of malachite green (MG; Szent-Gyorgyi et al., 2013), targeted to postsynaptic sites using the well-validated postsynaptic tag derived from the transmembrane and cytoplasmic region of mouse neuroligin-1.
* Molecular genetic, fluorescence-based approaches targeted to discrete cell types can enable automated detection and quantification of input-specific synapses in complex brain tissues.
* These fluorescence-based synapse labeling reagents can facilitate large-scale and cell-type specific quantitation of changes in synaptic connectivity across development, learning, and disease states.
* Future efforts should leverage volumetric imaging in cleared or expanded tissue for complete and high-resolution capture of the entire dendritic apparatus, application of additional molecular markers to distinguish different synapse types, and employ new presynaptic constructs for improved synaptic discrimination.
* Reagents can evaluate inhibitory synapse distribution across layer 2/3 (L2/3) pyramidal (Pyr) neurons using postsynaptic expression of a previously characterized, neuroligin-based construct.
Aided by automated image analysis, we quantitatively evaluated the distribution of inhibitory synapses identified from analysis of >90,000 synaptic puncta in neocortical Pyr neurons in superficial layers of somatosensory cortex. Using comprehensive fluorescence-labeling of cell type specific neurites in parvalbumin (PV), somatostatin (SST), and vasoactive intestinal peptide (VIP) Cre-driver transgenic mice allowed us to align FAPpost puncta to quantify inhibitory inputs for layer 2/3 (L2/3) Pyr neurons.
* These tools present a low barrier to use within the neuroscience community through volumetric confocal analysis of tissue specimens.
* The brightness of FAP/YFP post synaptic tags enable direct visualization of synapses in both live and fixed tissue without amplification, making them accessible tools for broad scale use.
* Synaptically-targeted fluorophores can be sparsely expressed in brain tissue, not just cultured neurons, to reveal properties of synaptic and input organization in a complex neural circuit.
* Fluorescence imaging enables use of multiple, spectrally distinct channels for cell type-selective identification of axonal inputs and specific molecules that can differentiate synapses.
* Volumetric data collection is rapid and requires only a confocal microscope, and images can be used for high-throughput, automated analysis.
* Reagents that separately enable visualization of excitatory and inhibitory synapses will also be useful tools for fluorescence-based quantitative imaging (Gross et al., 2013; Chen et al., 2015), if expression levels are high enough for reliable synapse detection.
* A critical challenge of these future possibilities will be the digital capture and storage of large anatomic datasets for computational analysis.
* The accuracy of true synapse detection from confocal images in sparsely-labeled tissue may also need to be calibrated for specific synapse types.
Kuljis et al. 2019, Fluorescence-Based Quantitative Synapse Analysis for Cell Type-Specific Connectomics, eNeuro. doi: https://doi.org/10.1523/ENEURO.0193-19.2019
YouTube video featuring Dr. Alison Barth: https://youtu.be/tXqTW-eVPas
Alison Barth, Professor
Carnegie Mellon University
FUNDING SOURCE(S)
NIH RF1MH114103

